Kaneka begins selling new coil for embolization of brain aneurysms in the United States
— Aims for 10 billion yen in global sales by 2023 —
Kaneka Corporation
October 22, 2020
October 22, 2020
In September this year, Kaneka Corporation (Headquarters: Minato-ku, Tokyo; President: Minoru Tanaka) began selling their new coil for embolization of brain aneurysms*1 (product name: i-ED Coil™) in the United States, after having released it in the Japanese market last year. Approval was obtained from the United States’ Food and Drug Administration*2 (FDA) in April 2020, and the product has been on sale from September through Kaneka Pharma America LLC. Kaneka plans to also expand the sales area to Europe and Asia and is aiming for 10 billion yen in sales of i-ED Coil™ by 2023.
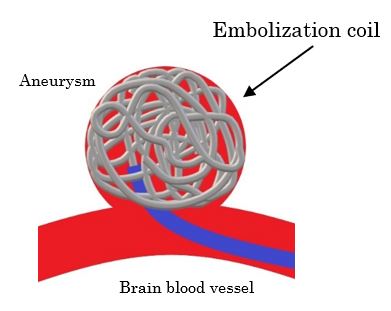
i-ED Coil™ is a new type of coil for embolization of brain aneurysms with the highest level of flexibility in the world*3 due to the thickness and structural ingenuity of the metal wire that it’s made from. This flexibility not only allows the coil to be more densely packed into the aneurysm compared with previous products, it’s also able to be used with aneurysms of irregular shapes. Its ability to help lower the risks of aneurysm rupture has been highly praised by doctors, and sales have been growing smoothly since its release in Japan in November 2019.
There are many people in the United States suffering from lifestyle diseases such as high blood pressure, hyperlipidemia and diabetes which can lead to cerebrovascular disease and cases of brain aneurysms are also increasing. In terms of treatment methods, each year there is a higher proportion of vascular IVR procedures performed, which are less of a burden on the body compared with surgery that removes part of the skull, and it’s predicted that the demand for coils for embolization of brain aneurysms will increase further.
From here on, Kaneka will continue to expand their lineup of products for treating cerebrovascular disease, which include embolization coils. They will also help solve a range of problems associated with cerebrovascular disease by providing solutions to domains outside of treatment, including diagnosis and prevention.
i-ED Coil™ is a new type of coil for embolization of brain aneurysms with the highest level of flexibility in the world*3 due to the thickness and structural ingenuity of the metal wire that it’s made from. This flexibility not only allows the coil to be more densely packed into the aneurysm compared with previous products, it’s also able to be used with aneurysms of irregular shapes. Its ability to help lower the risks of aneurysm rupture has been highly praised by doctors, and sales have been growing smoothly since its release in Japan in November 2019.
There are many people in the United States suffering from lifestyle diseases such as high blood pressure, hyperlipidemia and diabetes which can lead to cerebrovascular disease and cases of brain aneurysms are also increasing. In terms of treatment methods, each year there is a higher proportion of vascular IVR procedures performed, which are less of a burden on the body compared with surgery that removes part of the skull, and it’s predicted that the demand for coils for embolization of brain aneurysms will increase further.
From here on, Kaneka will continue to expand their lineup of products for treating cerebrovascular disease, which include embolization coils. They will also help solve a range of problems associated with cerebrovascular disease by providing solutions to domains outside of treatment, including diagnosis and prevention.
- A medical device used in treating brain aneurysms. It’s put through the inside of a catheter and sent inside an aneurysm in order to prevent blood from entering it. A brain aneurysm is a bulge that occurs in the arteries of the brain and if ruptured causes a subarachnoid hemorrhage.
- A governmental organization of the US in charge of approval or conformity control of products which consumers have contact within daily life, such as food or medicine in addition to cosmetics, clinical instrument, animal drug or toy.
- As found in research by Kaneka.