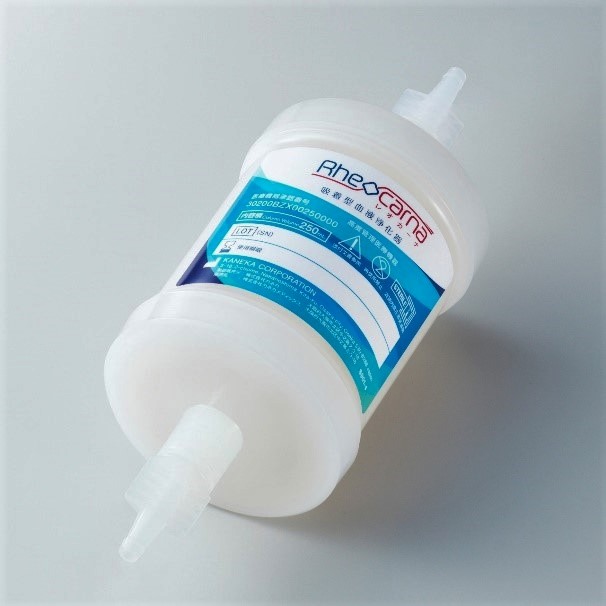
Approval acquired to manufacture and sell Adsorption Type Blood Purification Device for the most severe form of arteriosclerosis obliterans
Kaneka Corporation
October 13, 2020
October 13, 2020
Kaneka Corporation (Headquarters: Minato-ku, Tokyo; President: Minoru Tanaka) has developed a Adsorption Type Blood Purification Device (product name: RheocarnaTM) that treats severe form of arteriosclerosis obliterans (referred to below as ASO)*1 having lower limbs ulcer, and in August they acquired approval for its manufacture and sale. Once it is covered by health insurance, Kaneka will aim to begin retailing the product in the spring of 2021.
Due to the growing aging population and increasing diabetes and chronic renal failure, which lead to the most severe form, ASO patients are predicted to increase. The Adsorption Type Blood Purification Device developed by Kaneka work by selectively removing LDL-C*2 and fibrinogen*3 which are substances causing ASO symptoms and returns purified blood to the body, and thanks to the development of novel cellulose beads that adsorb the substances is now possible. This new way to treat ASO, a disease that leads to the most severe form and of which there are no other treatment*4, has had excellent results in clinical trial that evaluated healing rate of ulcer of RheocarnaTM*5.
Kaneka will continue into the future to accelerate the development of devices related to blood purification and ulcer treatment in order to offer solutions for the overall domain about ASO, including the field of inspection and diagnosis.
Due to the growing aging population and increasing diabetes and chronic renal failure, which lead to the most severe form, ASO patients are predicted to increase. The Adsorption Type Blood Purification Device developed by Kaneka work by selectively removing LDL-C*2 and fibrinogen*3 which are substances causing ASO symptoms and returns purified blood to the body, and thanks to the development of novel cellulose beads that adsorb the substances is now possible. This new way to treat ASO, a disease that leads to the most severe form and of which there are no other treatment*4, has had excellent results in clinical trial that evaluated healing rate of ulcer of RheocarnaTM*5.
Kaneka will continue into the future to accelerate the development of devices related to blood purification and ulcer treatment in order to offer solutions for the overall domain about ASO, including the field of inspection and diagnosis.
- A disease that causes arteriosclerosis where stenosis and occlusion of lower limbs artery occurred, leading to impaired blood flow (ischemia). In the early stages, it causes cold extremities, numbness and claudication. If it progresses further to become critical limb ischemia (CLI), which is accompanied by rest pain and ulcers, there is a higher risk of limb amputation or death.
- Low density lipoprotein-cholesterol. It is so called “Bad” cholesterol. Its role is to transport cholesterol produced in liver into the whole body. If increased too much, it can cause arteriosclerosis with symptoms of myocardial infarction, cerebral infarction, and ischemia of the lower limbs.
- It is a kind of blood clotting factor protein that is produced in liver.
- For critical limb ischemia (CLI), one treatment option is revascularization through bypass surgery or endovascular therapy. However, the existing therapies are not sufficiently effective in patients where the condition fails to improve and the ulcer becomes intractable.
- In clinical trial that evaluated the healing rate of severe ulcers that were prone to lead to lower limbs amputations, 45.9% of patients’ ulcers were able to be healed within 24 weeks from the start of the treatment.