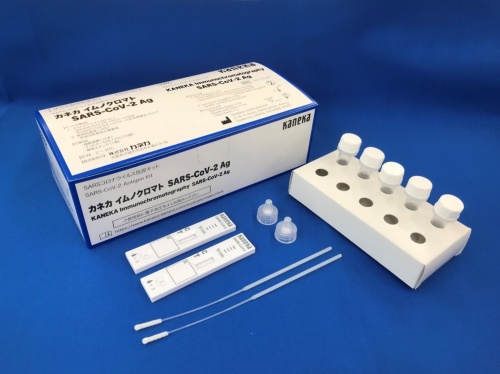
Kaneka Releases COVID-19 Antigen Test Kit
Kaneka Corporation
June 21, 2022
Kaneka Corporation (Headquarters: Minato-ku, Tokyo; President: Minoru Tanaka) has received authorization for the manufacture and sale of "Kaneka Immunochromatography SARS-CoV-2 Ag," a COVID-19 antigen test kit, and launched the new in-vitro diagnostic agent on June 15. The kit has a suggested retail price of 13,200 yen (for 10 tests), including tax.June 21, 2022
This diagnostic kit uses immunochromatography* to detect the presence of COVID-19 antigens in nasopharyngeal swabs and nasal cavity specimens in just ten minutes, using simple sample treatment, without the need for special testing equipment.
The global spread of COVID-19 has led to supply shortages for testing supplies such as antigen test kits in Japan. Kaneka is helping expand the COVID-19 testing system in Japan by providing a stable supply of test kits manufactured in Japan.
Kaneka has already released the KANEKA Direct RT-PCR Kit SARS-CoV-2, an in-vitro diagnostic agent that uses Kaneka's unique PCR technologies to produce test results in less than an hour, and KANEKA RT-PCR Kit SARS-CoV-2 (Omicron/Delta) ver. 2, which can detect multiple variants simultaneously. These products are contributing to faster testing in medical facilities, test centers and other sites. Kaneka will continue to expand its lineup of test kits as it works to address a broad range of infection-related issues through initiatives including performing commissioned manufacturing of DNA vaccine raw materials and intermediates, developing antibody drugs, and shipping vaccine using isothermal shipping packages.
*. Immunochromatography: A rapid testing method that uses capillary phenomena and antigen-antibody reactions. This method is exceptionally simple and speedy, capable of determining the presence of diseases in roughly 10 minutes.
Inquiry Form (KANEKA products for rapid genetic tests): www.kaneka-labtest.com/en/contact-us.html
Inquiry Form (KANEKA products for rapid genetic tests): www.kaneka-labtest.com/en/contact-us.html