Quality
Management Approach
Basic Policy
Aiming to benefit society and satisfy customers through a stable supply of safe and reliable products and services, Kaneka Group has set Quality Management Regulations to ensure thorough day-to-day quality control and product safety across the supply chain, from product design and development to delivery to customers.
Policy
Basic Policies for Responsible Care
-
Protect the natural ecosystem and reduce environmental impact
Focusing on the impact of corporate activities on the global environment and the ecosystem, we endeavor to reduce environmental impact and promote resource conservation and energy saving throughout the lifecycle of products. -
Offer safe products and information
We endeavor to offer products that are safe to distribute and use, and to provide adequate information on the products such as instructions on how to use and handle products correctly. -
Develop products and technologies in consideration of the environment and safety
Upon the development of new products, we give consideration to the environment and safety throughout the lifecycle of the products to the greatest extent possible, and endeavor to develop products and technologies with low environmental impact. -
Reduce waste and promote the recycling of plastics
We reduce waste associated with manufacturing and its processes. We actively develop technologies for the adequate disposal or recycling of plastic waste concerning our products in cooperation with relevant industries, and endeavor to dispose of and recycle waste in a proper manner. -
Enhance process safety, disaster‒prevention, and occupational safety and health
Safety and disaster prevention constitute the foundations of the local community’s trust, and occupational health and safety are issues that need to be fulfilled by chemical companies. We persistently strive to make improvements in these areas. -
Win public confidence
From the management to every employee, all our members shall act in compliance with laws, regulations, standards, etc. relating to environment and safety both at home and abroad. Our approach to Responsible Care as such shall be publicized accurately to the public, in hope of rightfully gaining public recognition and confidence.
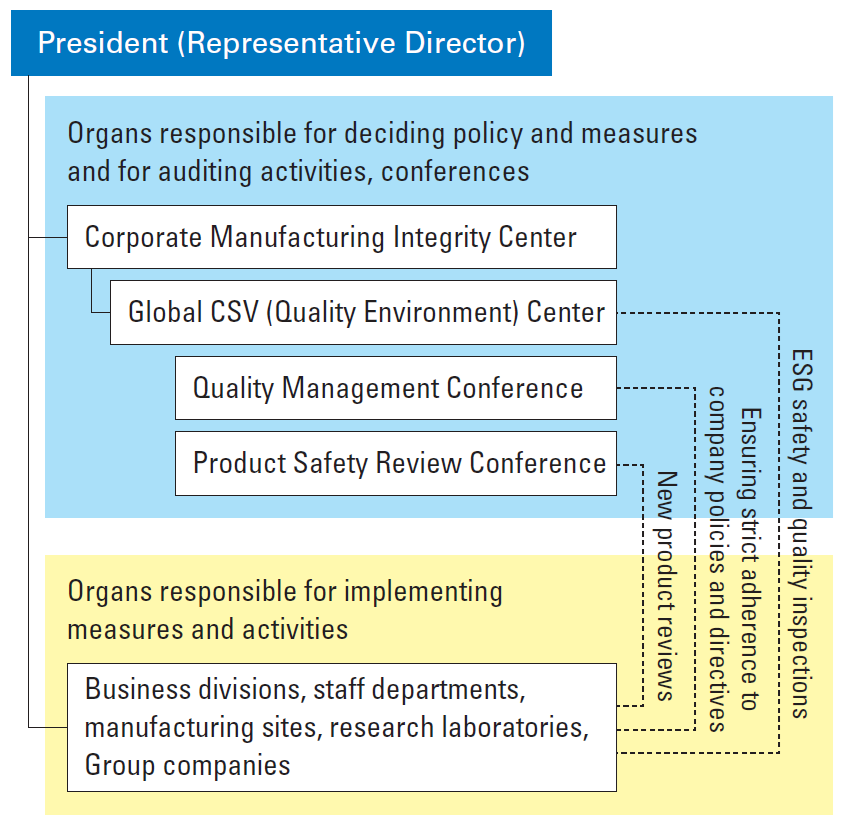
Promotion System
The Global CSV (Quality Environment) Center coordinates quality assurance activities throughout the Kaneka Group and drives quality management, including product safety assurance, at all stages from product design and development to raw material procurement, manufacturing, storage, transportation, sales, and disposal.
We hold quality management conferences, inviting personnel in charge of quality management in each business division, to instill Group-wide policies and instructions.
For new product launches, a Product Safety Review Conference meeting chaired by the Global CSV (Quality Environment) Center Director is held to ensure product safety.
We conduct ESG safety and quality inspections of business divisions, manufacturing sites, research laboratories, and Group companies to confirm their quality assurance efforts. Each of our businesses also undergoes regular third-party auditing and inspection and internal audits based on ISO 9001 and other established standards and regulations. In this way, we take steps to enhance our quality management system and thereby raise product quality standards.
Diagram of Promotion System
Targets and Performance
| Fiscal 2023 target | Fiscal 2023 performance |
|---|---|
| Inspection of Operation of Quality Management System |
|
| Ensuring thorough compliance with laws and regulations governing chemical substances |
|